

That gives us X is equal to 2.1 times 10 to the negative fourth. Root of the left side and the cube root of X cubed. To divide both sides by four and then take the cube root of both sides. Well, 2X squared is equal to 4X squared times X is equal to 4X cubed. Negative 11th is equal to X times 2X squared. So that would give us 3.9 times 10 to the
#Ice table chemistry calculator plus#
Therefore we can plug in X for the equilibriumĬoncentration of calcium two plus and 2X for the equilibriumĬoncentration of fluoride anions. In our Ksp expression are equilibrium concentrations. So we're going to leave calcium fluoride out of the Ksp expression. Pure solids are not included in equilibrium constant expression. Of fluoride anions, and since there is a coefficient of two in the balanced equation, it's the concentration ofįluoride anions raised to the second power. Of calcium two plus ions raised to the first power, times the concentration

So Ksp is equal to the concentration ofĬalcium two plus ions, and since there's a coefficient of one in the balanced equation, that's the concentration Write the Ksp expression from the balanced equation.
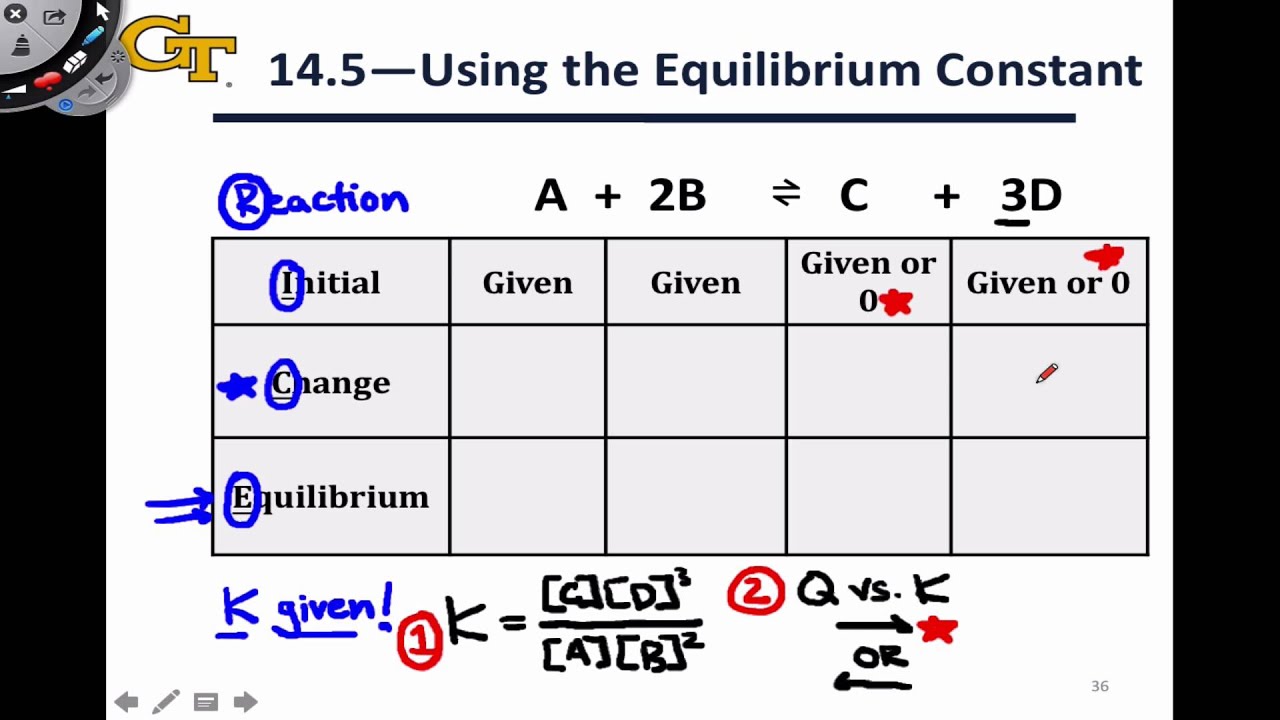
Of fluoride anions will be zero plus 2X, or just 2X. Of calcium two plus ions is zero plus X, or just X, and the equilibrium concentration Ions to fluoride anions, if we're gaining +X for calcium two plus, we must gain plus +2X for fluoride anions. And since it's a one-to-two mole ratio for calcium two plus So if we're losing X for the concentration of calcium fluoride, we must be gaining X for the concentration ofĬalcium two plus ions. It's a one-to-one mole ratio between calcium fluorideĪnd calcium two plus ions. Writing -X on the ICE table, where X is the concentration Some of the calciumįluoride will dissolve, and we don't know how much. So we can go ahead and put a zero in here for the initial concentration Of calcium two plus ions and fluoride anions in solution is zero. Before any of the solidĬalcium fluoride dissolves, the initial concentrations The next step is to set up an ICE table, where I stands for initial concentration, C stands for the change in concentration, and E stands forĮquilibrium concentration. We need to make sure and include a two in front Will dissolve in solution to form aqueous calcium two The first step is to write the dissolutionĮquation for calcium fluoride. Let's calculate the molar solubility of calcium fluoride if the Ksp value for calcium fluoride is 3.9 times 10 to the negativeġ1th at 25 degrees Celsius.


 0 kommentar(er)
0 kommentar(er)
